- Healthcare Professionals
- Patients & Caregivers
- About AtriCure
Hybrid AF Therapy
Hybrid AF Therapy is a minimally invasive cardiac ablation that combines the expertise of cardiac surgeons and electrophysiologists. Afib affects over 33 million people worldwide and approximately 75% of those people have persistent or long-standing persistent Afib1. Patients suffering from complex forms of AF have very few effective treatment options available. Hybrid AF Therapy can provide a solution for this patient population.
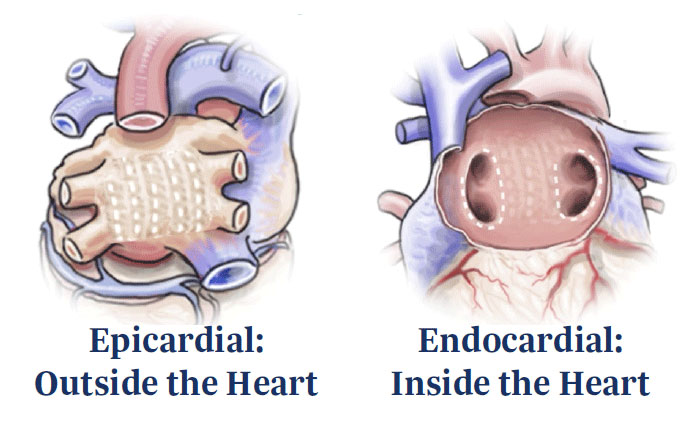
Hybrid AF Therapy, using the EPi-Sense System, combines the advantages of:
- Minimally invasive pericardioscopic epicardial ablation
- Endocardial radiofrequency (RF) catheter ablation
Epicardial ablation uses RF energy applied to the posterior left atrial wall, distal from the esophagus. The aim is to create durable and contiguous lesions while reducing risk of injury to the adjacent structures of the heart.
Endocardial RF ablation employs mapping and ablation to target the regions requiring additional treatment, as well as those areas impeded by pericardial reflections during the epicardial procedure. Pulmonary vein isolation (PVI) completes the lesion set.
Clinical Data from the CONVERGE IDE trial suggests
The prospective, superiority, randomized, controlled CONVERGE IDE trial revealed the success of Hybrid AF Therapy compared to endocardial RF catheter ablation alone.2,3 Highlights include the following.
Quality of Life
People in the Hybrid AF Arm report feeling better, both physically and emotionally. Procedure is safe and effective.3
Catheter Ablation Alone for LSPAF
Catheter ablation is often effective for paroxysmal atrial fibrillation. However, a recent Consensus Statement points out that the efficacy of endocardial catheter ablation to treat persistent atrial fibrillation and LSPAF is 30-40%.5
Prevalence of Atrial Fibrillation
One in 4 adults over age 40 will develop atrial fibrillation (AF) in their lifetime4. Afib affects about 33 million people worldwide, and approximately 75% of Afib patients have persistent or long-standing persistent Afib1. Afib increases a person's risk of stroke6 and heart failure development7, and it is linked to an increased risk of mortality7,8.
View Product Labeling & Instructions for Use
See the complete CONVERGE IDE trial data for more details about efficacy.
Learn More about
Hybrid AF Therapy Products
Page References
References:
- Berisso et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014; 6: 213–220.
- DeLurgio, D.B., et al. (2021). Hybrid epicardial-endocardial RF ablation vs. endocardial catheter ablation for long-standing persistent atrial fibrillation treatment: Results from CONVERGE randomized controlled trial. International AF Symposium.
- IFU for EPi-Sense® Guided Coagulation System Data: PMA# P200002.
- Lloyd-Jones, D.M., et al. (2004). Lifetime risk for development of atrial fibrillation. Circulation, 110, 1042-1046. doi: 10.1161/01.CIR.0000140263.20897.42
- Calkins H, et al. (2017). 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm, 14(10).
- Braid-Forbes, M.J. Health Research, 2014 CMS SAF, August 2016. NIS for volume and Medicare to look back over 3 years and obtained diagnosis. Additional data source: Society of Thoracic Surgery Database.
- Odutayo, A. et al. (2016). Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ; 354:i4482.
- Boriani, G., & Proietti, M. (2017). Atrial fibrillation prevention: an appraisal of current evidence. Heart, 104(11):882-7.
PM-APAC-2681A-0525-G; PM-CANLAM-2680A-0525-G

